From tissue to image – processing, sectioning, staining and labelling
The Histology Core Facility is more than a service. We are an integral part of your tissue imaging project and can guide you every step of the way.
We work closely with the Bioimaging Core Facility to ensure that your sample is appropriately processed for downstream imaging applications.
Traditional techniques such as paraffin embedding and histological staining are complemented with newer techniques such as cryo-sectioning and automated antibody labelling.
We reduce the need for thin sectioning with the use of tissue vibrotomes and whole tissue clearing.
In addition to The University of Manchester’s researchers, we also support external access.
How we work
More than cutting thin tissue sections
Histology is about getting your sample prepared in the best way for imaging.
Working closely with the Bioimaging Core Facility we understand what is needed to produce high-quality, quantitative images on tissue sections and whole tissues.
We offer two ways of creating excellent tissue sections:
User training
We provide full user training on the microtomes and cryostats.

Full service solutions
Our experienced histology technicians will process and section samples as a service to groups that do not have the time or the training.
Drop-off services
We also offer a range of drop-off services such as tissue processing, automated histological staining and automated antibody labelling.
Applications
Access the latest histological techniques
We are constantly investing in the facility to ensure that our users have access to the latest developments in histological techniques.
Advancements in tissue sectioning techniques, labelling technology and optical imaging, mean that we offer many of these new techniques alongside traditional paraffin and resin thin sectioning.
Small tissue samples
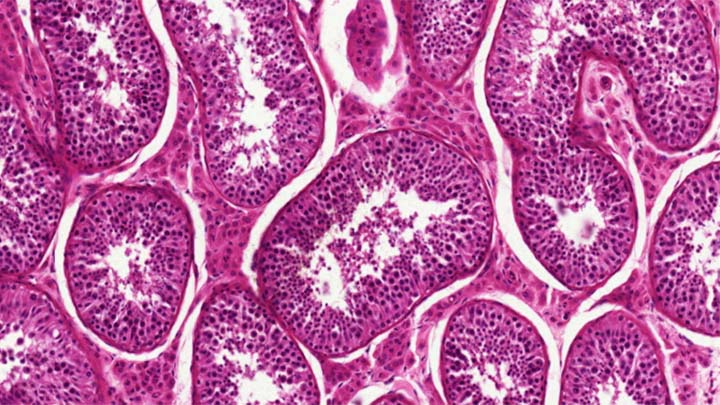
Paraffin embedding and sectioning of small tissue samples.
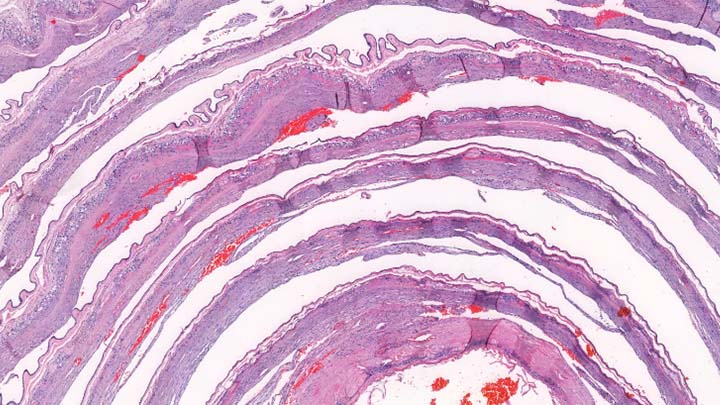
Cryo-sectioning
Cryo-sectioning of fixed frozen tissue samples.
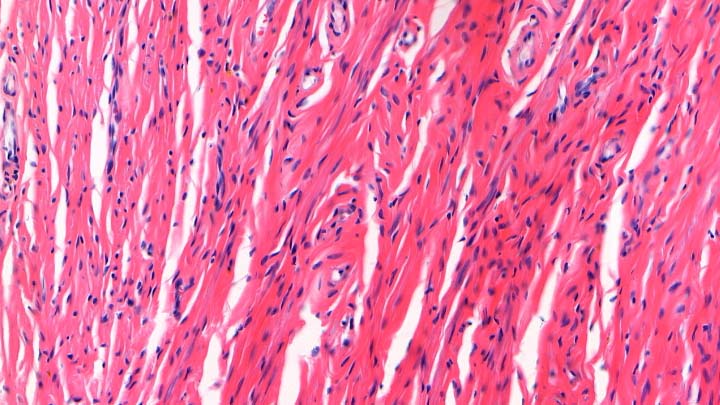
Vibratome thick sectioning
Vibratome thick sectioning of fresh tissue samples.
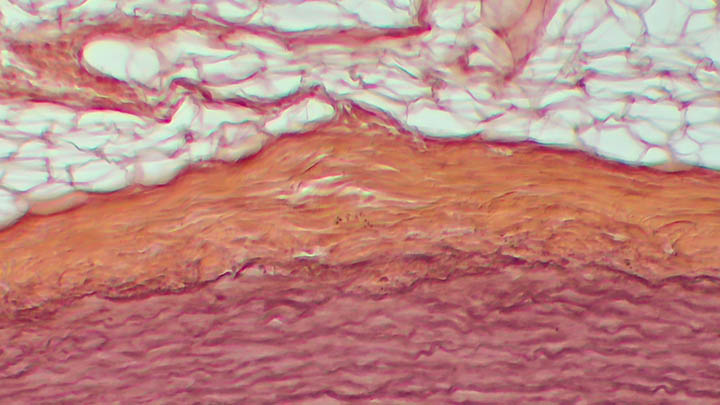
Automated histological staining
Automated histological staining such as H&E, Masson’s Trichrome and Picrosirius Red.
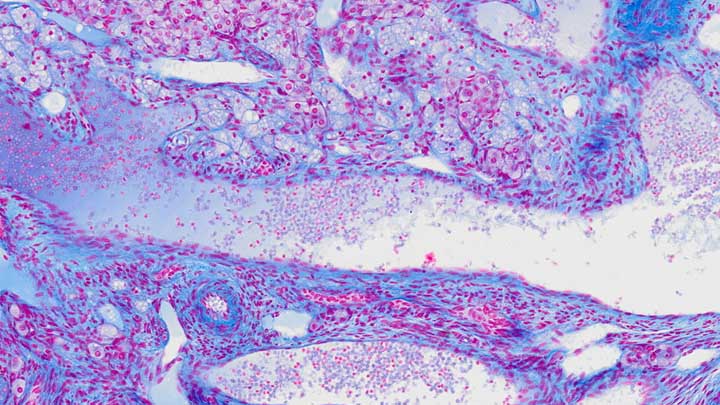
Automated IHC labelling
Automated IHC labelling for a range of cell markers using validated kits.
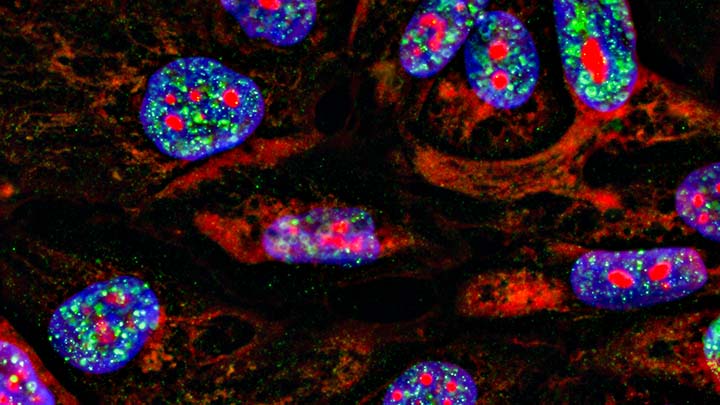
Whole tissue clearing
Whole tissue clearing to remove the need to section the tissue at all.

Multiplex imaging
Multiplex imaging through multiple rounds of antibody labelling, imaging and antibody stripping provides the ability to localise multiple protein markers within the tissue section. This multiplex spatial imaging provides a link between histology, bioimaging and the spatial transcriptomics available in the Genomic technology facility and Hyperion imaging system in the Flow cytometry facility.
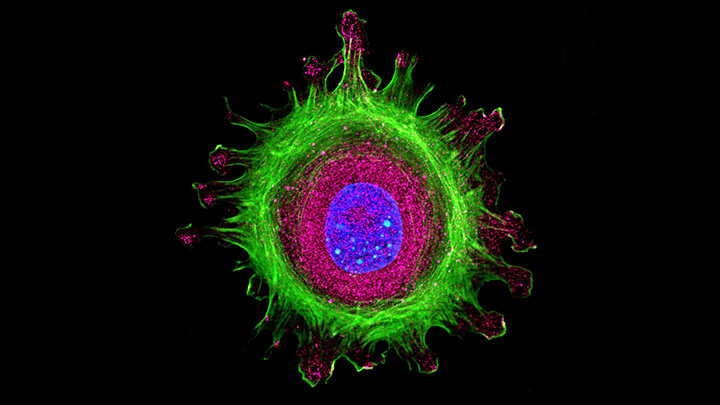
Technologies and equipment
Benefitting from investment
Investing in our equipment ensures that the Histology Core Facility remains at the cutting edge.
We are constantly investing in new equipment and technology to ensure that we can offer our users access to the latest and most appropriate techniques.
The tissue processors are run as a drop-off service and have a range of pre-programmed runs available. However, we are always flexible and can set up a new programme to meet your needs.
Cassettes are then transferred to the embedding centres ready for the user to paraffin embed the tissue samples in the desired orientation.
Traditional paraffin block thin sectioning is still one of the main techniques used by histologists.
We have a range of microtomes available within the facility and provide full and ongoing training to the user.
We are able to offer microtome sectioning as a service. Contact us for more information.
Cryo-sectioning overcomes many of the limitations of paraffin embedded tissue such as the need for antigen retrieval before IHC and the reduction of antibody sensitivity.
The technique itself is quite straightforward to understand but requires skilled training and experience to fully realise its potential.
We are able to offer cryo-sectioning as a service. Contact us for more information.
- 2 x Leica CM3050S
- 3 x Leica CM1950
We run the auto-stainer as a drop-off service and have a range of stains preloaded into the machine.
We are happy to configure new programmes or modify the timings of the existing ones to meet your requirements.
Stains offered include: H&E, Masson’s Trichrome, Picrosirius Red, FastRed/FastBlue, and Verhoeff-Van Giesons.
The automated staining system is connected to an automatic coverslipper providing a convenient solution for staining and mounting large numbers of slides ready for imaging in the Bioimaging Core Facility’s digital slidescanner.
The Leica BOND RX provides a convenient and reliable way of performing IHC, FISH, multiplexing and other labelling reactions.
The system uses validated reagents to automatically label the individual slides. There are a wide selection of reagents available.
Optical techniques such as confocal microscopy do not require very thin tissue sections such as those from a microtome or cryostat.
It is possible to cut fresh tissue on a vibratome and then fixed and label with fluorescent markers. The sectioning is then done optically on the confocal, providing excellent spacial information for techniques such as neurone tracking or vasculature mapping.
- 2 x Leica VT100S
Imaging depth in fluorescently labelled tissue is limited by the opacity of the tissue. The high lipid content of tissue restricts the amount of excitation light that can reach the fluorophore and reduces the amount of emitted light that can get back out of the tissue.
By using clearing techniques to remove the lipid it is possible to produce an optically transparent tissue which allows imaging millimetres deep into the sample.
This technique is excellent for understanding the three-dimensional nature of complex tissues such as the brain, lung and even whole organisms.
There are numerous clearing techniques and we provide access to a simple-to-use automated X-CLARITY clearing system.
Publications and outputs
Supporting high impact publications
High impact publications often rely on access to state-of-the art imaging systems. The Histology Core Facility provides those systems.
We are an essential resource that contributes to numerous high impact publications every year.
Here are some of the recent highlights.
Oncogene, 2021.
We show that high levels of ERK5 is a predictive marker of metastatic breast cancer using a combination of classical histological stains and specific antibodies to differentiate between the cancerous, normal and control tissue.
BMC Immunology, 2021.
This paper elucidated the role of β2 integrin in dendritic cell migration during infection with the nematode worm Trichuris muris. Paraffin wax sections were used for classical histological staining, while cryosectioning allowed preservation of tissue antigenicity to provide the ideal sample for immunofluorescent labelling with specific antibodies.
Nature, Scientific Reports, 2021.
Glucocorticoids (GCs) are widely prescribed anti-inflammatory medicines, but their use can lead to metabolic side-effects increasing food intake and modifying peripheral metabolism. As part of this study, wax sectioning was used for classical histological staining to look at lipid changes in tissue, and cryosectioning to provide the ideal sample for immunofluorescent labelling with specific antibodies against the glucocorticoid receptor and hypothalamic AgRP neurones.
Molecular Systems Biology, 2021.
Thick sections of fresh mouse trunk tissue were prepared on a vibratome before timelapse imaging on a confocal microscope. This provides 200um thick tissue, which is ideal for confocal imaging and ensured that the yellow fluorescent protein (YFP) in the tissue was unaffected due to the absence of any processing steps. Cryosectioning was then used to provide thin tissue sections for immunofluorescence labelling experiments.
External access
Working with other academic institutions and industry
Although primarily focused on supporting research at The University of Manchester, we are also able to support work from other academic institutions and industry.
Academic institutions
We are happy to provide access to all our histology equipment to users from other institutions.
We are also happy to provide a full histology service from tissue processing through to sectioning and staining.
It is also possible to extend this service through to whole slide scanning through our links with the Bioimaging Core Facility.
Contact us for further details.

Industry
We have worked for a number of multinational companies, both on an individual basis and as part of packages of work involving multiple facilities.
Work may be on a collaborative basis or purely as a service provision.
All work is fully documented and contractual, and non-disclosure compliance is assured.
Please contact our Business Development Manager for further details if you are interested in accessing the facility:
Dr Joanne Flannelly
Email: joanne.flannelly@manchester.ac.uk

Contact us
Find out more
Get in touch for further information or to inquire about using our facility.
Dr Peter March, Senior Experimental Officer
Email: peter.march@manchester.ac.uk
Telephone: +44 (0)161 275 1571
The Michael Smith Building
The University of Manchester
Oxford Road
Manchester
M13 9PT
Maps and travel
We are based in the Michael Smith Building (Building 71 on the University campus map).
Technology platforms
Technology platforms
We have a pioneering environment and facilities for research, innovation and technology development.
Technology platforms main page
